Rabeprazole sodium is a substituted benzimidazole and belongs to the class of proton pump inhibitors. Rabeprazole Sodium is an antiulcerant drug in the class of Proton Pump Inhibitors. It suppresses gastric acid secretion by inhibiting the gastric H+/K+-ATPase enzyme at the secretory surface of the gastric parietal cell. It is an enteric coated tablet, because of its coated formulation it is highly stable in stomach and because of higher pKa value of Rabeprazole Sodium it provides faster onset of action.
WHAT IS ATMOREB AND WHAT IT IS USED FOR?
• Rabeprazole tablets belong to a group of medicines called Proton Pump Inhibitors (PPIs).
• Rabeprazole tablets act by reducing the amount of acid made by the stomach.
Rabeprazole tablets are used to treat:
• Ulcer in the upper part of the intestine (duodenal ulcer) and benign stomach ulcer.
• Gastro-oesophageal reflux disease (GERD) with or without ulcer. GERD is commonly referred to as inflammation of the gullet caused by acid and associated with heartburn. Heartburn is a burning feeling rising from the stomach or lower chest up towards the neck. Rabeprazole tablets may be used as a long term treatment of GERD (GERD maintenance). Rabeprazole tablets may also be used for the symptomatic treatment of moderate to very severe gastro-oesophageal reflux disease (symptomatic GERD).
• Zollinger-Ellison Syndrome, which is a condition when the stomach makes extremely high amounts of acid.
Why is this medication prescribed?
Rabeprazole belongs to a group of medicines called ‚Proton Pump Inhibitors‚ (PPIs). They work by lowering the amount of acid that your stomach produces. Rabeprazole tablets can be used to treat the following conditions:
• Gastro-Oesophageal reflux Disease‚ (GERD), which can include heartburn. GERD is caused when acid and food from your stomach escapes into your food pipe (oesophagus).
• Ulcers in your stomach or the upper part of your gut (intestine).
• Zollinger-Ellison Syndrome where your stomach produces too much acid.
MK Medicine is a leading pcd franchise provider, contract manufacturer and hospital supplier of WHO-GMP certified Rabeprazole Sodium IP 20 mg Tablet
Symptomatic response to therapy with rabeprazole sodium does not preclude the presence of gastric or oesophageal malignancy, therefore the possibility of malignancy should be excluded prior to commencing treatment with rabeprazole.
No evidence of significant drug related safety problems was seen in a study of patients with mild to moderate hepatic impairment versus normal age and sex matched controls, the prescriber is advised to exercise caution when treatment with rabeprazole is first initiated in patients with severe hepatic dysfunction.
If you are pregnant, trying for a baby or breast-feeding.
If you have liver problems.
If you have any of the following symptoms: bleeding, difficulty swallowing, being sick frequently, or unexplained weight loss.
If you have ever had an allergic reaction to any medicine.
If you are taking any other medicines, including those available to buy without a prescription, herbal and complementary medicines.
Concomitant administration of clarithromycin with pimozide and cisapride is contraindicated. Contraindicated in patients with known hypersensitivity.
Headache
Upset stomach, mild diarrhea
Insomnia or nervousness; or
Rash or itching
Dizziness, confusion
Dry mouth
Stomach cramps
Nervousness
PO- Gastro-oesophageal reflux disease; erosive oesophagitis: 20 mg once daily in the morning for 4-8 weeks. Maintenance: 10-20 mg once daily.
Disclaimer:To be taken only after consulting with the doctor.
PHARMACOLOGY
Pharmacokinetics
Rabeprazole belongs to a class of antisecretory compounds (substituted benzimidazole proton-pump inhibitors) that do not exhibit anticholinergic orhistamine H2- receptor antagonist properties, but suppress gastric acid secretion by inhibiting the gastric H+, K+ATPase at the secretory surface of the gastric parietal cell. Because this enzyme is regarded as the acid (proton) pump within the parietal cell, rabeprazole has been characterized as a gastric proton-pump inhibitor. Rabeprazole blocks the final step of gastric acid secretion.
In gastric parietal cells, rabeprazole is protonated, accumulates, and is transformed to an active sulfenamide. When studied in vitro, rabeprazole is chemically activated at pH 1.2 with a half-life of 78 seconds. It inhibits acid transport in porcine gastric vesicles with a half-life of 90 seconds.
Absorption: Rabeprazole is acid-labile, it is administered as a delayed-release tablet so that it can pass through the stomach relatively intact. Once rabeprazole has left the stomach, absorption occurs within 1 hour of administration. The bioavailability is approximately 52%.
Distribution: Distributed in tissue, particularly gastric parietal cells.
Protein Binding: Very high; approximately 96% bound to human plasma protein.
Biotransformation: Rabeprazole is extensively metabolized in the liver by the cytochrome P450 enzyme system to 2 main metabolites. These 2 metabolites do not have any significant antisecretory activity.
Elimination: Normal renal function: Approximately 1 to 2 hours
Hepatic function impairment: 2 to 6 hours
Interactions
Rabeprazole increased gastric pH and has the potential to affect the bioavailability of any medication for which absorption is pH-dependent.
Combinations containing any of the following medications, depending on the amount present, may also interact with this medication.
• Digoxin(rabeprazole may increase gastrointestinal pH; concurrent use with rabeprazole resulted in increase of the serum peak concentration by 29% in normal subjects.
• Ketoconazole(rabeprazole may increase gastrointestinal pH; concurrent use with rabeprazole resulted in 30% reduction of bioavailability.
• Heartburn is often confused with the first symptoms of a heart attack. Seek emergency medical attention if you have chest pain or heavy feeling, pain spreading to the arm or shoulder, nausea, sweating, and a general ill feeling.
• You should not use this medication if you are allergic to rabeprazole or to similar medicines such as lansoprazole (Prevacid), esomeprazole (Nexium), omeprazole (Prilosec, Zegerid), or pantoprazole (Protonix).Rabeprazole is not for immediate relief of heartburn symptoms.
• Some conditions are treated with a combination of rabeprazole and antibiotics. Use all medications as directed by your doctor. Read the medication guide or patient instructions provided with each medication. Do not change your doses or medication schedule without your doctor‚s advice.
• Take this medication for the full prescribed length of time. Your symptoms may improve before the infection is completely cleared.
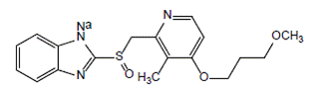
Rabeprazole sodium, which is a proton pump inhibitor. It is a substituted benzimidazole known chemically as 2-[[[4-(3-methoxypropoxy)-3-methyl-2-pyridinyl]-methyl]sulfinyl]-1H–benzimidazole sodium salt. It has an empirical formula of C18H20N3NaO3S and a molecular weight of 381.42. Rabeprazole sodium is a white to slightly yellowish-white solid. It is very soluble in water and methanol, freely soluble in ethanol, chloroform, and ethyl acetate and insoluble in ether and n-hexane. The stability of rabeprazole sodium is a function of pH; it is rapidly degraded in acid media, and is more stable under alkaline conditions. The structural Formula is:
| Pregnancy Category |
US: B (No risk in non-human studies) |
Legal Status |
UK: Prescription-only (POM) |
Routes |
Oral |
Important Notice:- The Database is still under development and may contain inaccuracies. It is not intended as a substitute for the expertise and judgement of your physician, pharmacist or other healthcare professional. It should not be construed to indicate that the use of any medication in any country is safe, appropriate or effective for you. Consult with your healthcare professional before taking any medication.


Pantoprazole 40 mg Tablet